|
PXL/HO/Cir-009/2020-21 Date:15.04.2020
Hyderabad
Dear Sir/Madam,
Sub: Advisory on Exports of Hydroxychloroquine & Paracetamol.
We appreciate the efforts you are putting in manufacturing the much-needed medicines for saving the lives across the nations at this global health crisis. We do worry about the hardships you are going through since last 5 weeks in these unprecedented times and we are leaving no stone unturned in resolving the issues faced by you.
You are aware that the Director General of Foreign Trade had imposed restrictions on exports of 13 APIs and its formulations on 03rd March 2020 (Notification No:50) and subsequently prohibited the exports Hydroxychloroquine and its formulations on 25th March 2020 (Notification No: 54 followed by Notification No:01, 04/4/20) in view of National Drug Security in the crisis of COVID-19 pandemic.
Pharmexcil has submitted multiple representations to the DGFT & other Government officials regarding the industry concerns w.r.t export restrictions and urged the Government for relaxation on restrictions. Considering the request of Pharmexcil & other industry bodies, DGFT has allowed relaxation on exports of 12 APIs and formulations, except Paracetamol on 6th Apr 20 (Notification No:2). On THE next day, the official spokesman of the Ministry of External Affairs has informed that the Government would license Paracetamol and Hydroxychloroquine (HCQ) in appropriate quantities to all our neighbouring countries who are dependent on our capabilities and the Govt will supply these essential drugs to nations who have been badly affected by the pandemic.
Having learned about the exports of some consignments of HCQ and Paracetamol to some nations with the consent of the Government, Pharmexcil has submitted representation on 13.04.2020 to the DGFT requesting to issue guidelines to the industry about the procedural formalities in getting the permission to export the formulations of Paracetamol and Hydroxychloroquine and are waiting for the guidelines.
It is learnt from couple of our member companies who have shipped the above products to USA & UK in the recent days, about the procedure followed by them and we would like to share the same for the benefit of exporters.
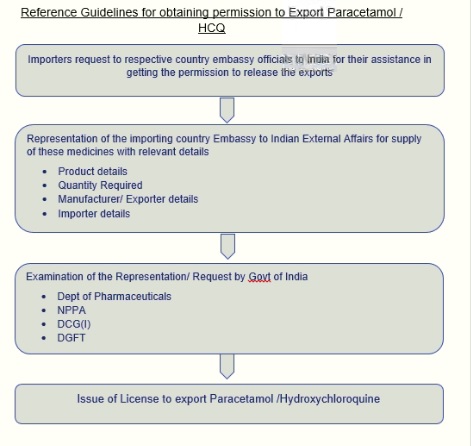
· It is learnt that the requests received by the Ministry of External Affairs, Govt of India from the Embassy/ Health Ministry/ other Government officials of the importing countries for the supply of these drugs would be examined on case to case by concerned departments and accordingly, the consignments may be released for export.
· Therefore Importing partners of the paracetamol & HCQ may please be requested the Embassy officials of their countries to send a detailed representation to Ministry of External Affairs(MEA), Govt of India (COVID19@mea.gov.in)specifying the need of country for these medicines, quantity required, details of Indian manufacturer/exporter as well as the details of importers. Details of Shipment(s) may be furnished In the DGFT proforma for processing permissions to export
· The request received by MEA will be assessed /examined by Dept of Pharmaceuticals along with Drugs Control General of India (DCG(I). Upon clearance from these departments, permission to Export will be granted to the exporter.
Please find the DGFT proforma for processing permissions to export. The contacts of the concerned departments are given for reference purpose.
· Ministry of External Affairs- covid19@mea.gov.in
· Dept of Pharmaceuticals- secy-pharma@nic.in
· NPPA- ssojha.77@gov.in
· Drugs Controller General of India - dci@nic.in
· Dept of Commerce- misra.shyamal@gov.in
· Director General of Foreign Trade- dgft@nic.in
In light of the non - availability of specific guidelines, we hope the information provided above may be of some help to the members to facilitate their exports.
With Regards
Udaya Bhaskar
Director General
Encl: Format |